Speaker
Description
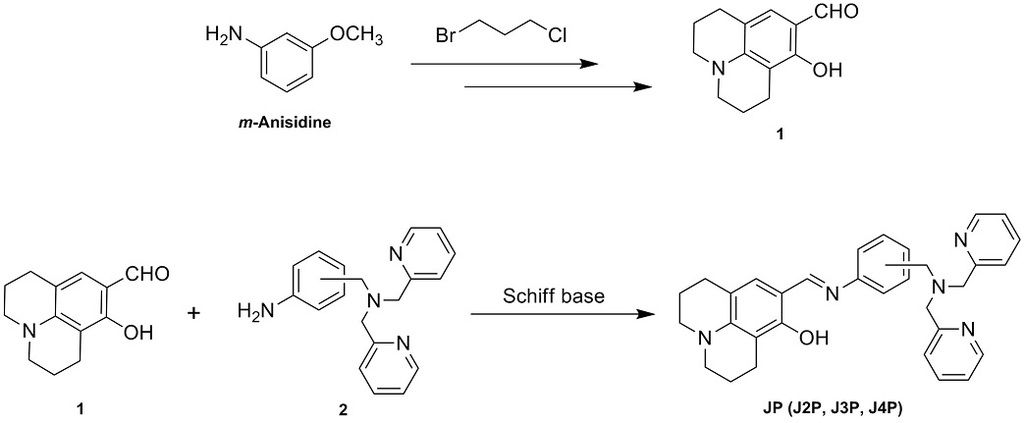
A new series of fluorescent sensors JP (J2P, J3P and J4P) was successfully synthesized via a simple Schiff base reaction by linking a fluorophore using 8-hydroxyjulolidinyl aldehyde 1 with di-(2-picolyl)amine (DPA) derivatives 2. The aldehyde 1 has been prepared by two important steps which are substitution of m-Anisidine with 1-bromo-3-chloropropane and Vilsmeier–Haack formylation reaction. On the contrary, substrates 2 were also synthesized by substitution of DPA to the corresponding nitrobenzyl bromide followed by the reduction of nitro group into amine group. All target compounds were characterized by using $^1$H NMR, $^{13}$C NMR, HRMS and UV-Vis spectroscopy. The maximum absorption wavelength (λ$_{max}$) of J2P, J3P and J4P in methanol were observed at 380, 415 and 420 nm, respectively. Based on the assumption that chelation-enhanced fluorescence (CHEF) may be involved in the sensing mechanism, the enhancement of the fluorescent signal(s) is expected to be investigated by fluorescence spectroscopy. And the sensing properties of JP(s) will be presented in details at the poster session.